The Molecular Compounds in Hemp - An Overview for Processors
A Processor's Guide to Understanding Hemp at the Molecular Level
Introduction
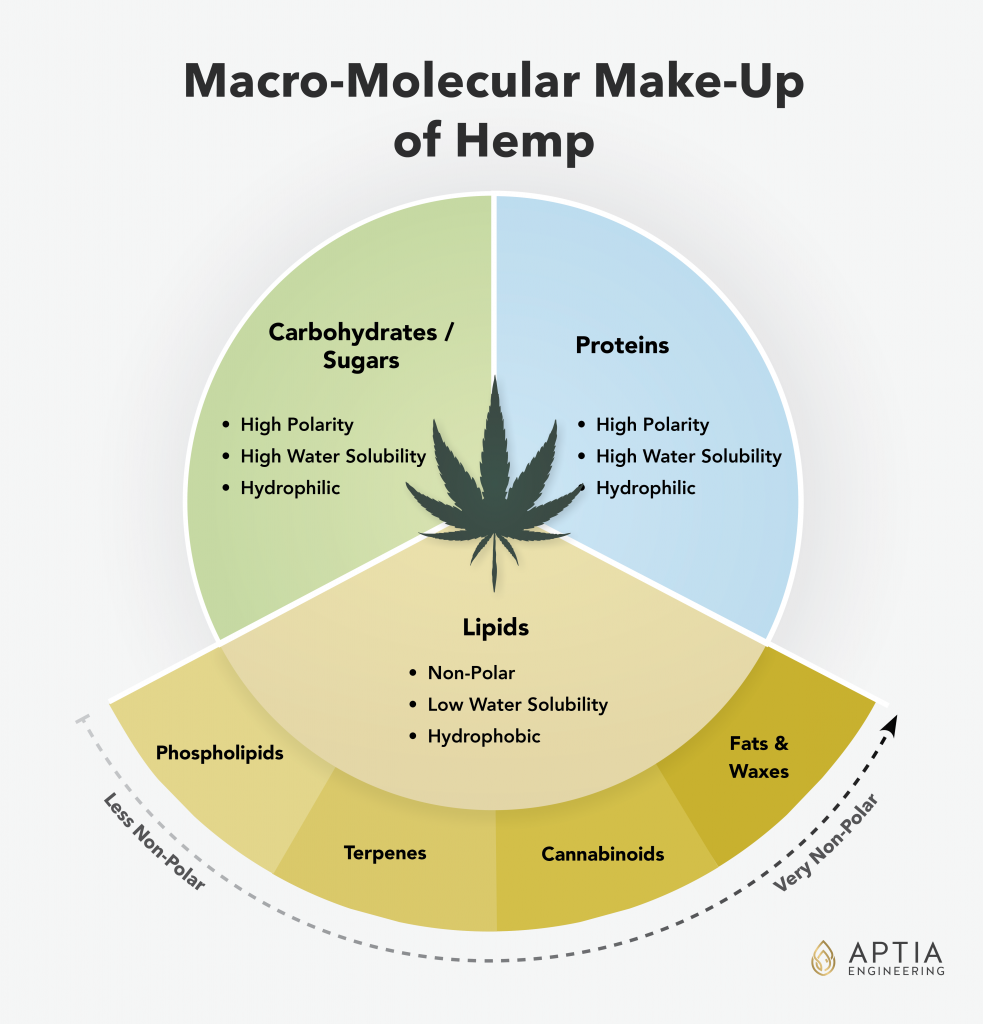
Hemp, just like any other natural botanical material, is comprised of a diverse array of molecular compounds. The large array of molecules can be grouped into a few key classes of molecules, with each class sharing similar chemical properties and characteristics. Understanding the basic classifications and general characteristics of each class is critical to understanding and evaluating the various extraction methods and technologies available today.
In hemp, there are compounds such as cannabinoids, gums, terpenes, fats & waxes, complex and simple sugars (carbohydrates), as well as structural and enzymatic proteins. Different solvents and extraction conditions interact with these groups of macromolecules differently, yielding extracts with dramatically different characteristics.
This article is dedicated to providing a high level overview of these key groups of molecules, and to explaining how the most common types of extraction interact with them.
“Different solvents and extraction conditions interact with these groups of macromolecules differently, yielding extracts with dramatically different characteristics.”
Among the characteristics of the key groups discussed, polarity is arguably the most influential. Polarity is a scale and unit of measure that is used to describe how “water-like” or how “oil-like” a particular substance is. Water is a highly polar molecule, while vegetable oil is a highly nonpolar substance. A key principle to understand in extraction chemistry is that “like dissolves like.” This means that one oily substance (like butter) will easily dissolve or mix with another (like vegetable oil), while polar substances dissolve in polar liquids like water. We explain each class of molecule below, starting with the molecules of the highest polarity.
Carbohydrates / Sugars
Characteristics
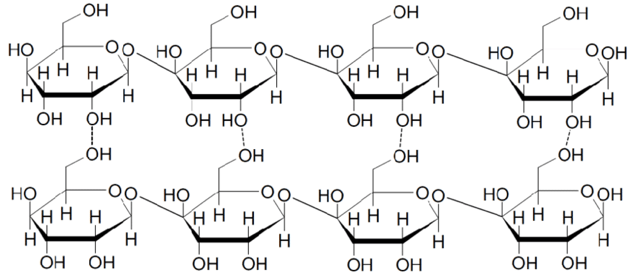
Carbohydrates, also called sugars, are one of the major groups of molecules that you are probably already familiar with. These compounds are made of one or more individual 6 carbon ring base structures called monomers. Simple sugars are composed of one or two of these monomers – familiar ones are Fructose, Glucose, and Sucrose (table sugar). These monomers can also be combined together to form long strands and large clumps, making compounds like starch or cellulose that you are also probably familiar with.
Short chain sugars are used for quick energy and food in animals and plants, and the long chain sugars are used for energy storage or as structural materials like plant walls.
Simple Sugars
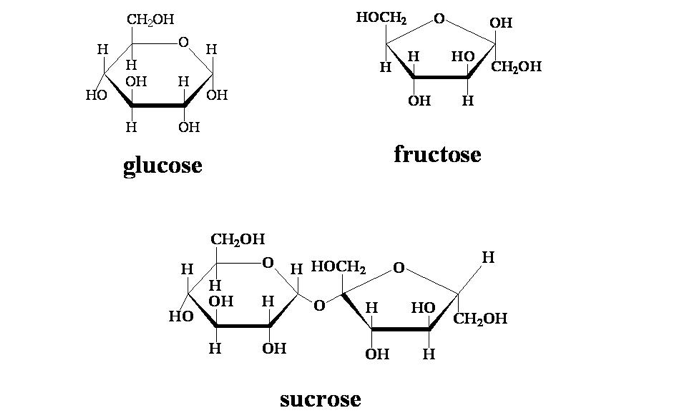
Complex Sugars
Impact on Extraction & Post-Extraction Processes
While sugar might be sweet and tasty, it is not something that you want to have in your extract. The botanical extractor seeks to leave the sugar undisturbed in the plant material during extraction. Sugars, both complex and simple, breakdown when heated. Sugar burns inside process equipment at high temperatures, causing charcoal-like “char” deposits which foul expensive machinery. These deposits increase the amount of regular maintenance required, and will ultimately break the equipment if they are not removed during cleaning. Making caramel from sugar on the stovetop is a common example of how sugar’s chemical structure changes when it is heated. Cleaning caramel out of a small frying pan is tough enough–cleaning sugar deposits out of a much larger piece of equipment can be very time consuming.

“Sugar burns inside process equipment at high temperatures, causing charcoal-like ‘char’ deposits which foul expensive machinery.”
Carbohydrates / sugars are highly polar and very water soluble. These compounds are hydrophilic. That means the less polar the extraction solvent, the smaller amount of sugar that will be extracted along with the fats and waxes.
Another important thing to know is that larger molecules like cellulose are generally harder to solvate than smaller molecules. So if an extraction solvent has some propensity for extracting sugar, then it will likely solubilize and extract much more simple sugar than complex sugar.
Sugars are highly polar, so water (also highly polar) is an excellent solvent for obtaining them from a plant matrix. An intermediate-polarity solvent like ethanol will also extract carbohydrates / sugars to a more limited degree. In contrast, non-polar solvents such as CO2, propane, butane, hexane, or heptane will extract a negligible amount of carbohydrates.
If sugars are extracted, then the operator may desire to remove these sugars before further processing of the extract is completed. A liquid/liquid separation method using water and a non-polar solvent is often used to remove sugar from extract before the extract is exposed to high processing temperatures.
Proteins
Characteristics
Proteins are responsible for most of the function of biological life. Proteins act as the individual molecular machines in the human body/factory that conduct all the reactions and transformations of materials that your body needs to function.
Proteins are generally only found in longer chains and large agglomerations in plants and animals, unlike sugars. This makes them less troubling during the extraction process because larger molecules are less easily extracted.
Proteins are hydrophilic, water soluble, and polar.
Protein Structure
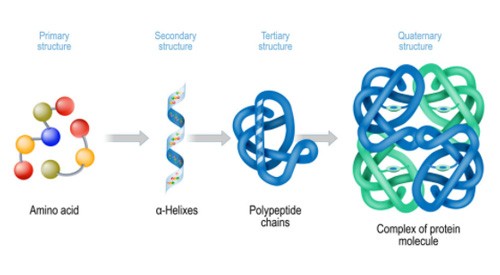
Impact on Extraction & Post-Extraction Processes
Proteins, like sugars, are subject to thermal breakdown. This can be seen in how a steak will char and stick to a grill or pan. Proteins in extract cause equipment fouling just like sugars do. Processors seek to leave proteins behind in the spent plant material during an extraction.
Proteins are very polar, and water is an excellent solvent for extracting both large and small protein molecules. An intermediate-polarity solvent like ethanol will extract proteins to reduced but still measurable degree. Non-polar solvents such as CO2, propane, butane, hexane, and heptane extract a negligible amount of protein so no protein based fouling is usually seen in these extracts.
Lipids
Characteristics
The lipid group contains all of the compounds that botanical extractors are interested in. Lipids are very non polar, so they dissolve well in oil-like substances, and they are sparingly or completely insoluble in water.
This lipid group includes waxes, fats, cannabinoids, terpenes, and gums. Since this is the most important group of molecules in botanical extraction, we will look at each of these groups in more detail below. Lipids in general are much more temperature stable than sugars or proteins, and with the exception of phospholipids, are not responsible for the fouling of equipment caused by their thermal degradation.
“The lipid group contains all of the compounds that botanical extractors are interested in.”
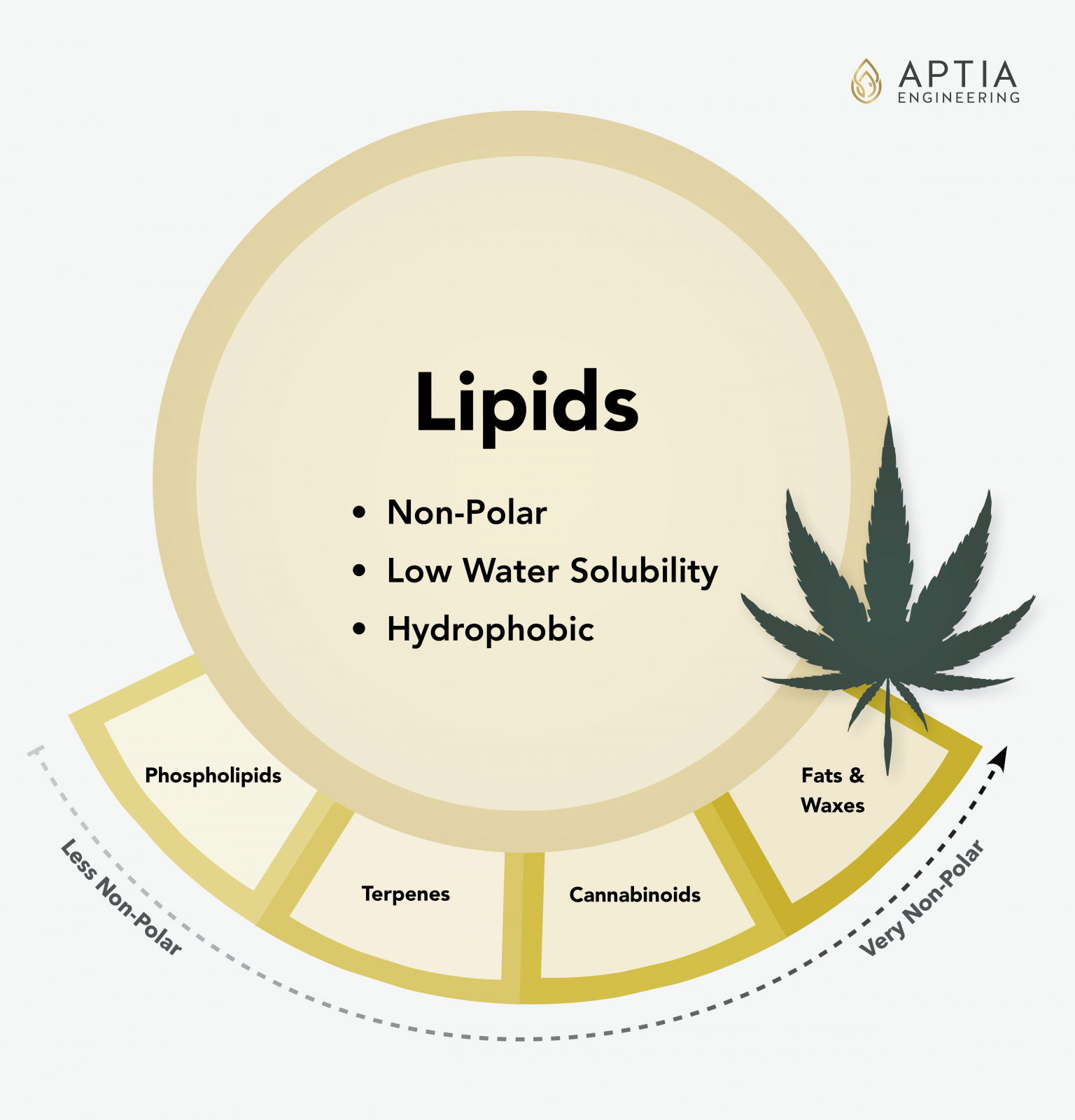
Lipids: Phospholipids (Gums)
Characteristics
Phospholipids, commonly called gums, are the most polar of the lipids. Each phospholipid molecule has a phosphatide “phospho” end that is water soluble, and a lipid end that is oil soluble. These compounds breakdown and foul equipment at temperatures above ~120 C, and they can also contribute to off-colors and off-aromas in extracts. These compounds are not desired in extracts.
Phospholipid Molecule
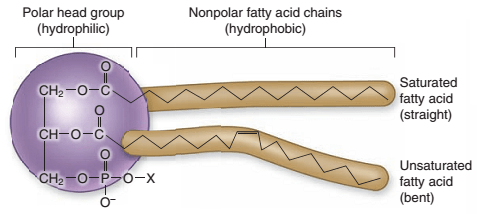
Impact on Extraction & Post-Extraction Processes
Phospholipid molecules are present in botanical materials and will be co-extracted to a degree that again depends on the extraction solvent and the extraction conditions. During extraction, the operator should leave these molecules undisturbed in the base plant material as much as possible.
If the operator does extract phospholipids, then these compounds are generally much more difficult to remove from the extract than sugars or proteins, because they are similar in structure and chemical characteristics to the desirable extract components.
Intermediate polarity solvents, such as ethanol, tend to extract more phospholipids than CO2 extraction or hydrocarbon extraction. Hydrocarbons and CO2 do extract phospholipids as well, but to a lesser extent which generally renders extract post processing easier and faster.
In order to remove phospholipids that have been co-extracted a “degumming” step will need to be implemented.
Lipids: Terpenes
Characteristics
Terpenes are generally substantially non-polar, and are generally of a smaller molecular size than cannabinoids, fats, or waxes. These compounds are responsible for giving hemp and other botanical materials their characteristic odors, and these compounds are often referred to as “essential oils.” Terpenes do not mix well with water or other non-polar solvents, and form an oily layer at the top of a bucket of water if you try to suspend them in a water solution. These molecules have also been suggested as having synergistic qualities when used with CBD to increase the effects and benefits of CBD use. These compounds do not cause fouling in equipment, are generally co-extracted to a large extent in most extraction processes, and are a desirable component of many extracts.
Examples of Popular Terpenes in CBD



Impact on Extraction & Post-Extraction Processes
Terpenes that have pleasant odors are highly valuable to botanical extractors. Since these compounds are non-polar small molecules, mid-polarity solvent such as ethanol, as well as non-polar solvents such as hydrocarbons all do a nice job of extracting them. Since terpenes are small molecules, they have lower boiling points than the majority of the extract, and tend to be lost after extraction during solvent recovery and other higher-temperature processes because the terpenes vaporize when heated and then are hard to recover. To further complicate matters, some terpenes can undergo breakdown at elevated temperatures, which ruins their value and creates off-odors.
To adequately protect these compounds, it is necessary to limit the temperatures that terpenes are exposed to during solvent recovery or other post extraction processes. Terpenes are easier to retain in extraction processes that use solvents with lower boiling points, such as propane, butane, or CO2. A substantial quantity of the terpenes are usually lost during processes that use higher boiling point solvents such as ethanol, hexane, or heptane.
Lipids: Cannabinoids
Characteristics
Cannabinoids are a group of compounds which are naturally occurring in hemp plants. CBD is one of these cannabinoids. Cannabinoids are larger molecules than the aromatic terpenes discussed above, and are even more non-polar. Cannabinoids are also very temperature stable and have high boiling points. Extracting and purifying cannabinoids is the goal of most hemp processors.
CBD – Cannabidiol
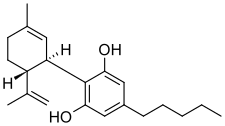
Impact on Extraction & Post-Extraction Processes
Hemp extractors want to extract as much of the available cannabinoids as possible from the plant material, and they usually wish to achieve the highest concentration of cannabinoid in the finished extract that they can. These factors are at odds with each other, because a more efficient extraction extracts more cannabinoid, but it also extracts more fats/waxes and everything else as well. This results in a lower potency when compared with a less efficient, but more selective extraction.
Ethanol, propane, butane, hexane, heptane, CO2 and many other solvents all are excellent extraction solvents for cannabinoids. That means that they extract cannabinoids out of the plant material very efficiently.
Lipids: Fats / Waxes
Characteristics
 Fats and waxes are the most non-polar group of compounds encountered in hemp and other botanical extractions. These compounds are extremely temperature stable and thus do not foul equipment. You can easily remember this by thinking about how you lubricate or season a pan by adding oil or butter to it before cooking. During cooking, these lipids even help prevent other fouling compounds from sticking to your cookware. Despite their excellent thermal stability, fats and waxes are not desired in extracts. They cause extracts to appear cloudy or hazy at room temperature, which makes them less appealing to consumers. An extract that contains fat will also have reduced cannabinoid, or active botanical ingredient, concentration.
Fats and waxes are the most non-polar group of compounds encountered in hemp and other botanical extractions. These compounds are extremely temperature stable and thus do not foul equipment. You can easily remember this by thinking about how you lubricate or season a pan by adding oil or butter to it before cooking. During cooking, these lipids even help prevent other fouling compounds from sticking to your cookware. Despite their excellent thermal stability, fats and waxes are not desired in extracts. They cause extracts to appear cloudy or hazy at room temperature, which makes them less appealing to consumers. An extract that contains fat will also have reduced cannabinoid, or active botanical ingredient, concentration.
Impact on Extraction & Post-Extraction Processes
Being the most non-polar of all the compounds, fats and waxes are extracted to the largest extent by non-polar solvents such as CO2, hexane, or heptane. Warm temperatures also increase the extraction of fats and waxes because many of these compounds are highly viscous or gelatinous at room temperature. Colder extraction temperatures and higher polarity solvents reduce the amount of fat and wax extracted from plant material.
Depending upon the amount of fat and wax that is extracted along with the cannabinoids, a secondary purification process called winterization may be required to remove them from the extract. This process is generally easier to do than liquid/liquid washing extract to remove protein or sugar. It is also generally an easier process to conduct than degumming.
In Summary
Arriving at a final, pure CBD product is a process of separating desired compounds from plant material. Due to the complex molecular composition of the plant, multiple steps are required to separate the range of unique compounds in hemp material and create a final refined product.
Understanding the macro-molecular structure of plant feedstock, and specifically how solvents interact with these compounds, is a key precursor to selecting the ideal solvent for your application and developing a successful processing methodology.
To learn more about the basic scientific principles of solvent extraction, check out our resource on the topic here.
Additional Resources
If you enjoyed this overview, you might also enjoy this beautifully rendered journey into a leaf, which provides the biological rather than chemical close-up of a common leaf.
- Published by Aptia Engineering on February 2, 2021