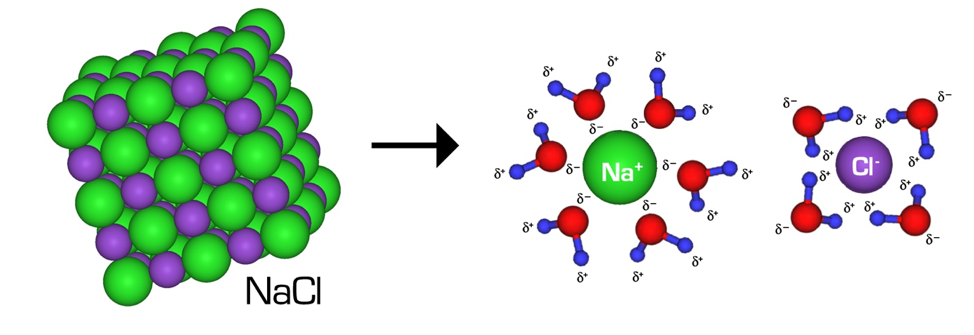
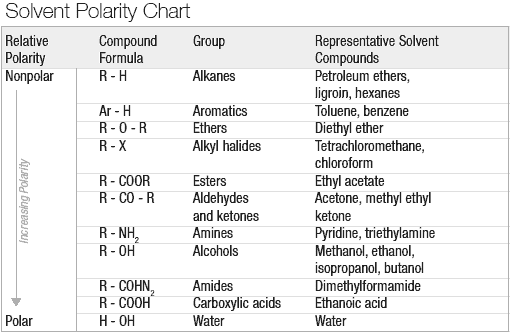
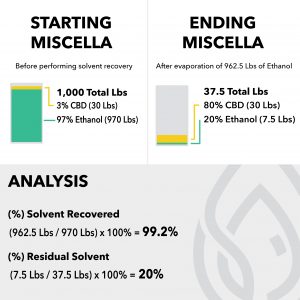
At the broadest level, the two methods of botanical extraction are solvent extraction and mechanical extraction. This article focuses on solvent extraction, but it is important to understand the basic principles and differences between the two techniques.
Mechanical extraction refers to an extraction that is caused by mechanical force pressing a liquid out of a solid material. This method is usually reserved for either high value oils or for botanicals sources which have very high ratios of oil to solids. Extra virgin olive oils are produced in this manner inside giant screw presses. Citrus essential oils are produced in a similar manner by pressing citrus peels. Some mechanical extraction methods use heat to increase extraction efficiency.
The benefits of mechanical extraction include eliminating the need for flammable solvents and protecting temperature sensitive compounds. The downside of mechanical extraction is the extraction efficiency is much lower than solvent extraction. Even the most efficient expellers leave about 7% residual oil in the base material.
In hemp extraction, mechanical extraction is an acceptable method if:
- Either the market value of the oil is so high it can cover the loss of some cannabinoids in the plant material, or
- You are going to process the mechanically extracted material afterwards, recovering the residual oils in the plant materials.
Mechanical extraction is not advised if you are concerned about the loss of roughly 50% or more of the oil material that would be left in the spent material following extraction. Overall, mechanical extraction is suitable for extraction of fewer materials in fewer applications than solvent extraction.
The second method of extraction, and the focus of the rest of this article, is solvent extraction, which has much wider applications and higher possible efficiency than mechanical extraction. The multitudes of available solvents with distinct characteristics make the extraction of diverse compounds possible.
Solvent extraction provides higher throughput, lower energy consumption, and higher extraction efficiency than mechanical extraction.